Headache Toolbox: Onabotulinum A (Botox)
At least 2% of the population suffers from chronic migraine, adisorder that can be very disabling in terms of pain, quality of life, missed workdays, and interruption of usual activities throughout the month. In October 2010, Onabotulinumtoxin A (onabot) brand name Botox (Allergan, Irvine, CA, USA) was approved by the US Food and Drug Administration (FDA) as a preventive strategy for patients having headaches most days of the month, lasting at least 4 hours per day. This approval was based upon 2 randomized, placebo-controlled trials conducted at 122 sites across North America and Europe that demonstrated decreased number of headache days, decreased hours of headache, and improved function with administration of onabot.
Chronic migraine, per the latest edition of the International Classification of Headache Disorders (ICHD-3 beta), is defined as headache at least 15 days per month, with a least 8 of those days meeting criteria for migraine, in this pattern for more than 3 months. This means that for at least 8 headache days, light sensitivity and noise sensitivity, or nausea, must be present, and the pain should be moderate to severe in intensity. However, the prescribing information for onabot approved by the FDA did not put all these criteria in place. Instead, chronic migraine, for the purposes of approved use of onabot, is described simply as headache (with any characteristics) at least 15 days per month lasting 4 hours per day. Onabot is not approved, nor has it been proven to work, in individuals with headaches fewer than 15 days per month.
Onabot is an injectable protein produced by a bacterium (Clostridium botulinum) that paralyzes muscles into which it is injected. The precise location and quantity of each injection has been tested extensively for safety and effectiveness in treating a wide variety of disorders. Onabot is believed to work for migraine by blocking pain-signaling transmission between the head and neck and the central brain where migraine is generated.
Onabot is not a cure for migraine. In fact, in the trials leading to its approval, there were only about 2 fewer headache days per month in those who received it compared with those who received placebo, although the number of hours of headache per month was decreased by about 1/3. However, people who had received onabot in the studies were found to be better able to function and perform their usual activities even when they did have headache.
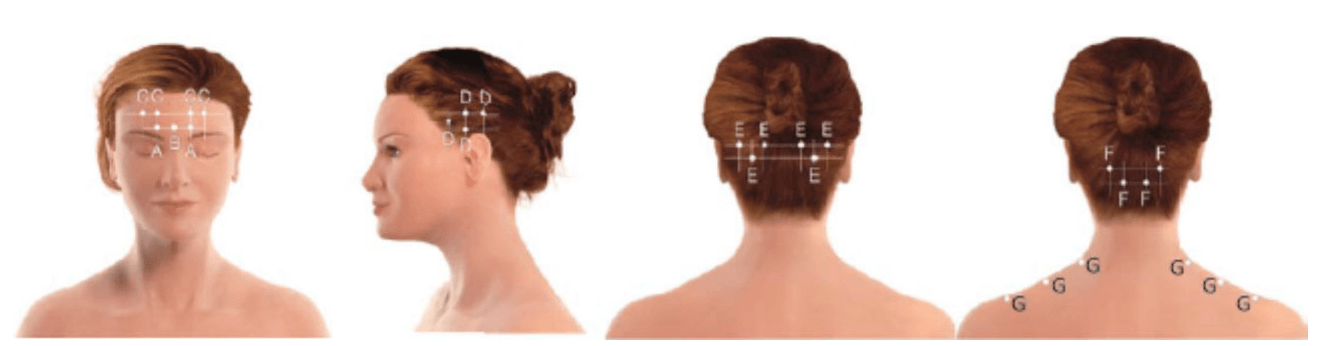
The 2 clinical trials that led to FDA approval used a standardized set of injections called the PHASE III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) protocol. With this protocol, developed and tested extensively, 31 small injections of 5 units each are placed at prescribed locations over the forehead, sides of the head, and back of the head and neck. The injections are just under the skin, creating a small bubble or wheal at the site that is usually not visible beyond a few hours. The PREEMPT injection sites are illustrated in the Figure.
The amount of medicine approved by the FDA for chronic migraine prevention, and administered in the PREEMPT protocol, is 155 units. However, onabot only comes in vials of 100 or 200 units. Rather than throw out the remaining 45 units in the bottle, many practitioners will offer to administer the remainder in areas in which patients particularly have pain. This additional treatment strategy is called “follow the pain,” and it was also used by many of the PREEMPT testing sites before FDA approval. Unfortunately, although “follow the pain” injections are frequently administered, it is not fully established whether they provide additional benefit.
The PREEMPT protocol for onabot injections is the only FDA-approved injection pattern for chronic migraine, and practitioners are specially trained in its administration. Although cosmetic onabot is chemically identical to that used for chronic migraine, the amounts and locations tested and approved for headache treatment are very different from that used for other indications.
Onabot in general is well tolerated and usually is without systemic side effects. However, about 9% of people report neck pain, 5% headaches, and 4% may have a temporary drooping of the eyelid called ptosis. About 3% will experience muscle pains, and 2% will have some facial muscle paralysis, eyebrow elevation, or muscle spasms. All of these are temporary should they occur.
Patients typically notice they cannot wrinkle their forehead after onabot injections, and when they resume being able to do this, it can be a sign that the drug is wearing off. The effectiveness of onabot tapers off at 3 months, sometimes sooner. If there are side effects, they typically are much shorter in duration than the 3 months of effect on headache.
There are some people who have neurologic muscular diseases who need to be observed closely for more severe side effects if onabot is administered. Allergies to onabot are uncommon, but as with any medicine, they are possible, and range from a local reaction to one case of severe allergy and death, thought to be possibly related to another medicine that was mixed with the onabot. Other isolated reports of trouble breathing, speaking, and swallowing have been reported, but these events seemed to occur in patients being injected with onabot in larger amounts for different problems and were not reported in the large studies for chronic migraine.
Onabot has not been tested in pregnancy and therefore should not be administered to pregnant women or in women who may become pregnant in the 3 months after it is administered. It was not tested in those under 18 years of age for chronic migraine and therefore is not indicated for this younger group.
The entire injection process takes about 10-15 minutes, and afterwards, patients are able to drive home and resume normal activities. Vigorous neck exercises, hair dyes, and permanents are discouraged for 24 hours after the procedure.
Onabot works as an effective preventive intervention in many migraineurs. When it works for chronic migraine, the results can be dramatic, not just in reducing headache days, but reducing all disabling aspects of daily headache. For this reason, it is important to keep a diary of headache days, intensity, and duration both prior to and after receiving the drug. Patients may take 4 weeks after injection to notice benefit, although many see improvement sooner. There is good evidence that when it works, onabot has a cumulative effect, with better and better response with each cycle administered every 3 months across a year. Therefore, patience is a virtue and trying onabot for 2–3 cycles may yield benefit not seen with just one set of injections. However, after 2–3 sets of injections, if no improvement is noticed, onabot should probably be discontinued. For those who do respond, injections are continued every 3 months. To test whether it can be discontinued, the injections are spaced further apart and if the headaches do not increase, the onabot may be stopped. After stopping onabot a headache diary should be continued to assure that there is no increase in migraine frequency, intensity, or duration without the drug.
Chronic migraine is an important problem for at least 2% of the population, having an adverse impact upon an individual’s quality of life, as well as that of their families. Onabot is the first approved intervention found to result in a significant improvement in this disorder. While it does not result in cure, it represents a breakthrough in effective treatment.
Deborah Tepper, MD
Cleveland Clinic Headache Center
Cleveland, OH, USA